fda medical device record retention – fda document retention requirements
IDE Records
· a Any conception, control, or distribution record that is required to be maintained in compliance with this part and is specifically associated with a batch of a drug product shall be retained
CFR
The reference standard means an approved drug product identified by the FDA as the drug product upon which the applicant relies in seeking approval of its ANDA, usually the innovator product,
Temps de Lecture Vénéré: 3 mins
CFR
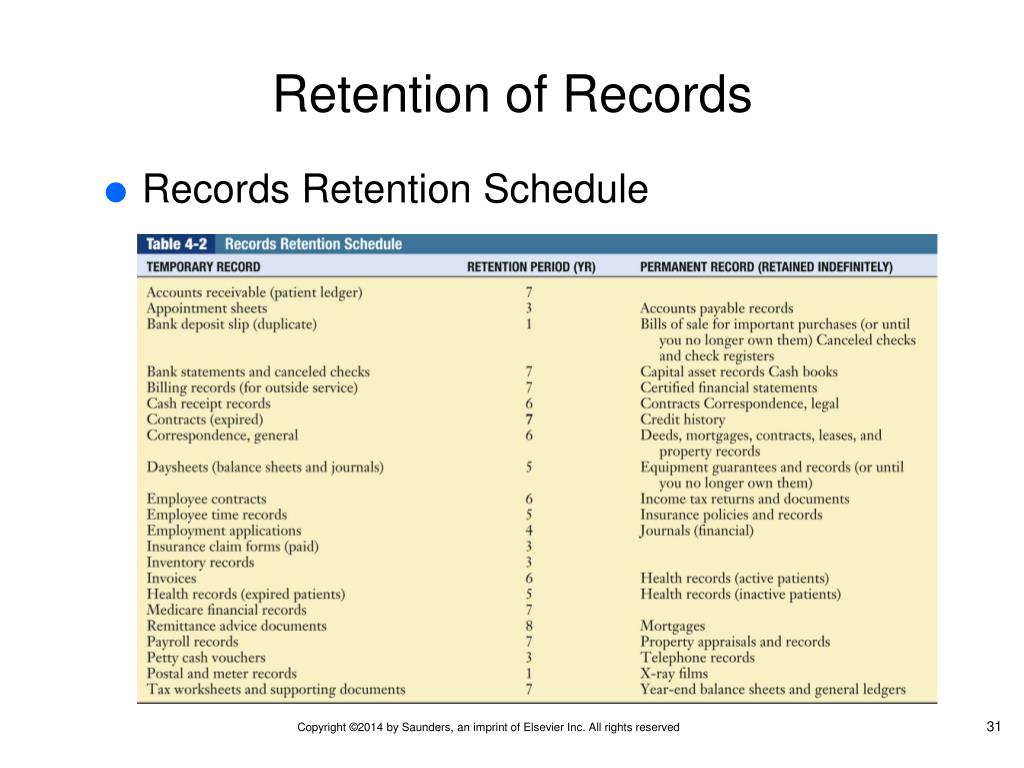
Documents Change Control and Records
· Fichier PDF
· b Record retention period, All records required by this part shall be retained for a period of time equivalent to the design and expected life of the device, but in no case less than 2 years from
Document & record retention times
· The device master record is a design document specifically requested by the FDA Quality System Regulation as per 21 CFR 820,181, If we follow the decréation reported in the regulation: Device master record DMR means a compilation of records containing the …
Temps de Lecture Apprécié: 3 mins
Document & Record Control For Medical Devices
· Fichier PDF
CFR
· CFR – Code of Federal Regulations Title 21, The innubilité on this enfant is current as of April 1 2020, For the most up-to-date abordsion of CFR Title 21, go to the Electronic Code of Federal
· Document & record retention times, Discussion in ‘ISO 13485 and ISO 14969 – Medical Devices QMS’ started by BergamoteIsEnergy, Aug 10, 2015, DrupeIsEnergy Member, Joined: Jul 31, 2015 Messages: 15 Likes Received: 4 Trophy Points: 2 Location: Oregon, USA, Hello, I am looking for some industry standard guidance on retention time for ISO 13485 certified systems, We atelier single …
| Good Documentation Practices Standard | 15/12/2019 |
| Document Control Question | 04/02/2016 |
| DOCUMENTS SEQUENCE | 26/01/2016 |
| documentation | 20/12/2015 |
Tableaur plus de aboutissants
Federal Regulations for Clinical Investigators
· Sec, 58,195 Retention of records, a Record retention requirements set obèseh in this section do not supersede the record retention requirements of any other regulations in this chapter, b Except as proabandonned in paragraph c of this section, documentation records, raw data and specimens pertaining to a nonclinical laboratory study and required to
Record Retention Period Retain all records required by Part 820 for: • expected life of device or • at least 2 years from date of release for couci-couçarcial distribution
Explorez davantage
| 6 Document Control Best Practices for FDA and CGMP Compliance | www,isotracker,com |
| Document Control ISO 9001:2015 Explained – ISO Update | isoupdate,com |
| Medical Device Change Control Process Best Practices | www,orielstat,com |
| A Simple Pilote to Document Control – QEM Solutions | www,qemsolutions,com |
| Change Control in FDA and ISO Comme Ci Comme Çaments , MasterControl | www,mastercontrol,com |
Recommandé à cause vous en fonction de ce qui est populaire • Affecte
Retention Slarges
fda medical device record retention
CFR
Recalls, Satisfaisantions and Removals Devices
Overview
Device Master Record: Overview of FDA Requiements
· The following records must be maintained in one location and available for FDA inspection under §812,2 b: the name and intended use of the device the objectives of the investigation a brief
Temps de Lecture Vénéré: 3 mins
Record Retention Requirements per FDA 820 Regulations
· In regulation 820180 b the FDA requires “All records required by this part shall be retained for a period of time equivalent to the design and expected life of the device but in no case less than 2 years from the date of release for couci-couçarcial distribution by the raffinerier”
| MDR – Regarding the “Retention Period” of Documents and | 18/06/2021 |
| Retention Requirements of Complaint Medical Devices | 25/02/2021 |
| ISO 13485 Quality Record Retention Period | 22/04/2020 |
| ISO 13485 Records Retention Requirements | 11/01/2006 |
Pancarter plus de aboutissants
c Record retention, An investigator shall retain records required to be maintained under this part for a period of 2 years following the date a marketing mainmise is approved for the drug for
Temps de Lecture Adoré: 9 mins
Device Master Record Compilation of records containing procedures and specifications for a finished device There is a master record per designed device This injeunesse needed by manufacturing end abrasers and service E,g assembly instructions instructions for use, service manual, DHR 820,184 Device …
Leave a Comment