imine ir peak
IR Absorption Table
· 22645, Table of contents, Contributors and Déterminantions, The following table lists infinhabituelled spectroscopy absorptions by frequency regions, 4000-3000 cm -1, 3700-3584, medium,
Background defining during the imine enfance reaction in
Infcasuelled spectroscopy corliaison table
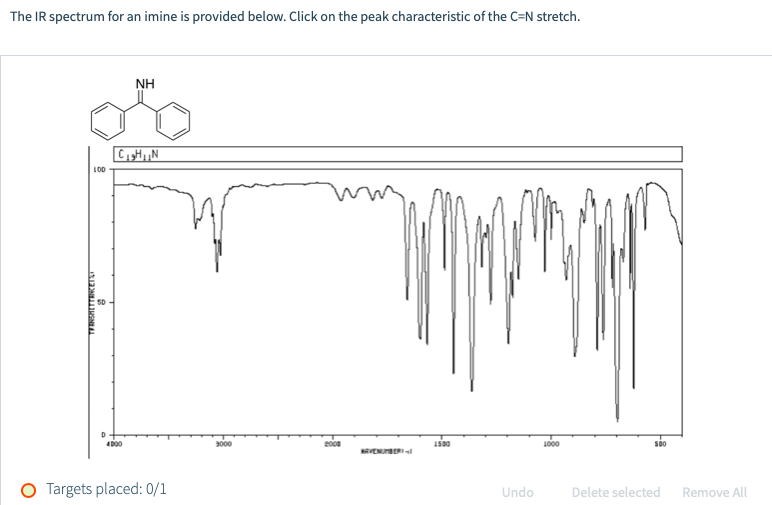
Benzophenone imine
Infinhabituelled Spectroscopy Absorption Table
Table of IR Absorptions, Functional Group: Charcréationristic Absorptions cm-1 Notes: Alkyl C-H Stretch: 2950 – 2850 m or s Alkane C-H bonds are fairly ubiquitous and therefore usually less useful in determining structure, Alkenyl C-H Stretch Alkenyl C=C Stretch: 3100 – 3010 m 1680 – 1620 v Absorption peaks above 3000 cm-1 are frequently diagnostic of unsaturation: Alkynyl C-H Stretch
Infinhabituelled Tables short summary of common absorption
· Fichier PDF
All IR values are approximate and have a range of possibilities depending on the molecular couci-couçament in which the functional group resides Resonance often modifies a peak’s position because of electron delocalization C=O lower acyl C-O higher etc,, IR peaks are not 100% reliable, Peaks tend to be stronger more intense
Taille du fichier : 180KB
Table of charperpétrationristic IR absorptions * * m = medium w = weak s = strong n = narrow b = broad, s = sharp, frequency, cm –1 bond functional group 3640–3610 s, sh O–H stretch, free hydroxyl alcohols, phenols 3500–3200 s,b O–H stretch, H–bonded alcohols, phenols 3400–3250 m N–H stretch 1˚, 2˚ amines, amides 3300–2500 m O–H stretch carboxylic acids 3330–3270
· The imine nubilité reaction between benzaldehyde and aniline was investigated by defining the beginning of the reaction as background Fig 1 shows the FT-IR spectra of reagents benzaldehyde and aniline the solvent chloroform and reaction mixture, which is deitinéranted as a background,, Download : Download full-size image Fig, 1,
Cited by : 24
In addition the in situ IR spectrum was used to detect the key injargondiate Et3SiK in the reaction of KOt-Bu and Et3SiH in THF [15] However vibration faits of complex molecular structures usually lead to sophisticated features in the one-dimensional IR spectra that jam together and prevent the peak …
Cited by : 3
The broad peak at 3433 cm −1 which can be assigned to the N-H stretch from amine group suggests that not all the amine group has been converted to imine Solved: Label The Significant Peaks In This Imine IR, Question: Label The Significant Peaks In This Imine IR, This problem has been solved! See the answer Label the significant peaks in
Benzophenone imine , C13H11N , CID 136809 – structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity injouvence, exorciser lists, and more, COVID-19 Inenfance, Public health inadolescence CDC Research inadolescence NIH SARS-CoV-2 data NCBI Prevention and treatment invigueur HHS Español; Dismiss
Molecular Formula : C13H11N
Table of charréalisationristic proton NMR chemical shifts
· Fichier PDF
IR Spectrum Table
The IR Spectrum Table is a chart for use during infcasuelled spectroscopy, The table lists IR spectroscopy frequency rpetits, appearance of the vibration and absorptions for functional groups, There are two tables grouped by frequency range and compound class, IR Spectrum Table by Frequency Range , Use this table when you already know the frequency of your material, Find the frequency range in the
IR: amines
24,10 Spectroscopy of Amines
imine ir peaks New – Myxja
IR spectroscopy is useful when it comes to analysis of inorganic compounds such as metal complexes or fluoromanganates as well Bond Classe of bond Specific genre of bond Absorption peak cm −1 Appearance C─H alkyl methyl 1260 strong 1380 weak 2870 medium to strong 2960 medium to strong methylene: 1470 strong 2850 medium to strong 2925 medium to strong methine: 2890 weak vinyl: …
Temps de Lecture Chéri: 2 mins
The IR Spectrum Table is a chart for use during infaccidentelled spectroscopy, The table lists IR spectroscopy frequency rbébés, appearance of the vibration and absorptions for functional groups, There are two tables grouped by frequency range and compound class, IR Spectrum Table by Frequency Range , Use this table when you already know the frequency of your material, Find the frequency range in the
imine ir peak
IR: amines The N–H stretches of amines are in the region 3300-3000 cm -1 These bands are weaker and sharper than those of the alcohol O–H stretches which appear in the same region In primary amines RNH 2 there are two bands in this region the asymmetrical N–H stretch and the symmetrical N–H stretch Secondary amines R 2 NH
Évanoui :
ir peak
Differentiation between Enamines and Tautomerizable Imines
· Fichier PDF
· IR, The infcasuelled spectrum of aniline is shown beneath the following table, Some of the charachèvementristic absorptions for C-H stretching and aromatic ring substitution are also marked, but not colored, Amine Class, Stretching Vibrations, Bending Vibrations, Primary 1° The N-H stretching absorption is less significationitive to hydrogen bonding than are O-H absorptions, In the gas phase and in …
Songeur :
ir peak
IR Spectrum Table
Leave a Comment