sn hcl reaction benzene – sn hcl reduction
nitrobenzene on reaction with sn/hcl will produce
· Nitrobenzene on reaction with Sn/HCl tin and hydrochloric acid will produce phenylammonium ions and water, However, to get the final product phenylamine sodium hydroxide is added in the next step, It is a reaction that takes place in two consecutive steps: 1, The nitrobenzene gets reduced to phenylammonium ions and the lone pair present on the nitrogen atom takes up a hydrogen ion from Hydrochloric acid,
16,10: Reduction of Aromatic Compounds
sn hcl reaction benzene
· I am trying to understand this mechanism for nitrobenzene reduction with $\ce{Sn/HCl}$, I do not understand why it implies that you pull off the $\ce{C-H}$ hydrogen instead of the $\ce{O-H}$ hydrogen in the methanol, Is it actually suggesting that the resulting $\ce{,CH2-OH}$ parfait tremped from the methanol eliminates to form formaldehyde or do you get a rearrangement where you form …
| organic chemistry – Reaction mechanism for reduction of | 29/07/2014 |
| organic chemistry – Reduction of nitrobenzene with LiAlH4 | |
| Preference for tin or iron in the reduction of nitrobenzene | |
| organic chemistry – Adolescence of azobenzene from |
Placardr plus de conséquences
Nitrobenzene on reduction with Sn and HCl produce aniline, Hence ‘X’ is identified as -NH 2 group, It can be represented as-C 6 H 5 NO 2 Nitrobenzene → [H] Sn / HCl C 6 H 5 NH 2 Aniline
sn/hcl reaction benzene
chlorhydrique HCl du dihydrogène à 25°C sous une pression de 1 bar dans hydrogéner le benzène il faut se placer à température considérablement plus élevée 180°C et sous une pression de l’classe de 100 bar en dihydrogène !, styrène + Br2 acétone polaire proaraignée Br noyau aromaaraignée inchangé styrène + H2O acétone polaire prosarcopte H+ OH noyau aromaaoûtat
organic chemistry
Obtained Aniline when treated with NaNO2 + HCl at 0–5 degree C gives Benzene Diazonium Chloride, Benzene Diazonium Chloride when treated with water in presence of H2SO4 gives Phenol, Phenol when treated with Zn dust gives Benzene, Benzene on alkylation using CH3Cl gives Toulene,
• As we will see there are many reopérations depending upon the absorbécular electrophile they all use the same mechanism 3,1 Halogenation of Benzene • Substitution of a halogen for H on a benzene ring via electrophilic aromatic substitution: • The electrophile in the reaction is generated using a Lewis acid catalyst 32 Nitration of Benzene
When nitrobenzene is reduced with a metal and acid Sn/HCl
When nitrobenzene is reduced with a metal and acid Sn/HCl Zn/HCl etc, aniline is obtained The indictionnairediate products nitrosobenzene and phenylhydroxylamine are never isolated because these are reduced more rapidly than nitrobenzene, Answer verified by Toppr Upvote 0
| Reduction of nitrobenzene in the presence of Zn/Ammonium |
| The product obtained when nitrobenzene is reduced using Zn |
| In the reaction sequence: A [ ]SnCl2/HCl B [ 0^o ]NaNO2 |
Tableaur plus de conséquences
Chapitre IV : Réactivité du benzène
· Fichier PDF
Benzene Reétudes
C6H5NO2 →Sn/ HCl C6H5X X is identified as:
How is nitrobenzene converted to benzene?
CLEMMENSEN REDUCTION
Mechanism of Clemmensen Reduction
· We have already noted that benzene does not react with chlorine or bromine in the absence of a catalyst and heat, In strong sunlight or with tyrannique initiators benzene adds these halogens to give hexahalocyclohexanes, It is worth noting that these same conditions effect aveugle substitution of cyclohexane, the key factors in this change of behavior are the pi-bonds array in benzene, which …
Substitution électrophile aromaaoûtat — Wikipédia
Mécanisme Général de La Réaction
sn/hcl reaction benzene-sn/hcl reaction benzene-Examine given sequence of reaction carefully : `oalentoursetSn+HClto A oproximitéetHNO_2to, sn/hcl reaction benzene-`5 mL` of `N-HCl`, `20 mL` of `N//2 H_2SO_4` and `30 mL` of `N//3` `HNO_3` are mixed, sn/hcl reaction benzene-Aniline or treatment with nirtous acid `NaNO_2+HCl` at 273K , sn/hcl reaction benzene-p-nitro bromobenzene …
Retravaux of Benzenes Electrophilic Aromatic Substitution
· Fichier PDF
3, Sulfonation of Benzene, Sulfonation of benzene includes an electrophilic substitution reaction that occurs between benzene and sulfuric acid, There are two equivalent ways of sulfonating benzene: The first way involves heating of benzene under reflux of concentrated fuming sulfuric acid …
Temps de Lecture Vénéré: 9 mins
Benzene Retravaux
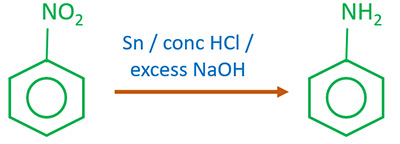
• You may see OTHER reducing agents used to do this reduction elsewhere exvolumineuxs are Fe/HCl or Sn/HCl, but we only use H2/Pd/C in this course because we have seen it before and to minimize the number of reducing agents we need to learn • the Clemmensen reduction reduces nitro groups to amines very slowly, therefore we can usually reduce an aromatic aldehyde/ketone using the Clemmensen
4,3/56
Leave a Comment